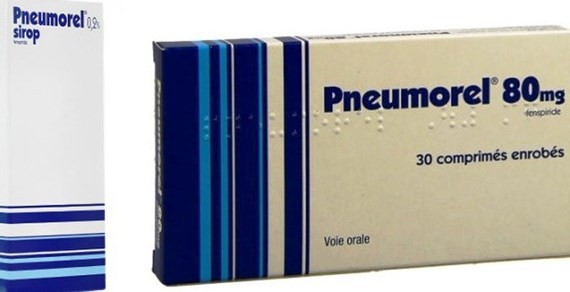
The National Agency for Medicines (ANSM) believes that the drug fenspiride carries the risk of cardiac arrhythmia.
After receiving the information that French health authorities have withdrawn the drug from the market, the Vietnamese medicine watchdog also announced to discontinue sales of syrup and tablet Pneumorel imported by Phytopharma Company used for cough treatment because of a cardiac risk.
The Administration requested Phytopharma company in coordination with distributors to withdraw the medicine from retailers and wholesalers. Departments of health in cities and provinces must announced withdrawal of Pneumorel to all drug stores and infirmaries in its jurisdiction as well as supervise the withdrawal activities.
The withdrawal decision of the drug must be posted in departments of health websites.
Presently, France has banned the use of the drug for its cardiac risk.
























